botiss maxgraft®
– Processed human allograft
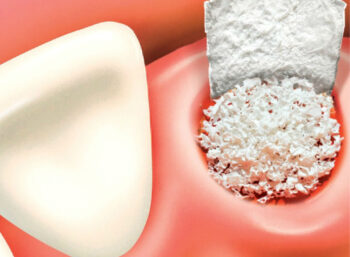
maxgraft® is a sterile, high-safety allograft product, derived from human-donor bone, processed by Cells+Tissuebank Austria (C+TBA). C+TBA, a high-quality bone bank, is regulated, audited, and certified by the Austrian Federal Office for Safety in Health Care and fulfills the highest EU safety standards.
For experienced oral and maxillofacial surgeons, allograft bone blocks for block augmentation are the only real alternative to harvesting the patient’s own autologous bone. This helps preventing well known risks such as donor-site morbidity, infection, post-operative pain, and bone-stability loss. The excellent biological regeneration capability of maxgraft® results in a predictable clinical outcome.
Properties
- Preserved biomechanical properties
- Sterile – no antigenic effects
- Storable at room temperature for five years
- Osteoconductive properties supporting natural and controlled tissue remodelling
Indications
- Implantology
- Periodontology
- Oral and CMF Surgery
maxgraft® granules
- Localized augmentation of the ridge for future implant placement
- Ridge augmentation
- Osseous defects
- Extraction sockets
- Elevation of maxillary sinus floor
- Repair of intrabony periodontal defects
maxgraft® blocks
- A predictable and highly effective alternative to traditional block grafting
- Ridge augmentation